Posts: 2417
Threads: 5
Joined: January 3, 2018
Reputation:
22
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 9, 2020 at 6:59 pm
(This post was last modified: June 9, 2020 at 7:07 pm by polymath257.)
(June 9, 2020 at 12:42 am)Paleophyte Wrote: On a side note, googol and googolplex have always failed to impress me. They're stunt numbers based on the number of fingers on your hands. If really big numbers is all you want then 4^^4 is a bit better than 50% more digits than a googolplex
I thought about this a bit more and it is wrong. 4^^4 is about 10^10^154. A number with 50% more digits than a googolplex would be about
10^(1.5*10^100), so far less than 10^10^101 (which would have 10 times as many digits as a googolplex). The number 4^^4 is about a googolplex to the power of 10^50, so (googolplex)^\sqrt(googol)
By the way, 10^^10 is called Decker.
Posts: 1307
Threads: 3
Joined: November 16, 2018
Reputation:
18
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 10, 2020 at 12:53 am
(June 9, 2020 at 6:59 pm)polymath257 Wrote: (June 9, 2020 at 12:42 am)Paleophyte Wrote: On a side note, googol and googolplex have always failed to impress me. They're stunt numbers based on the number of fingers on your hands. If really big numbers is all you want then 4^^4 is a bit better than 50% more digits than a googolplex
I thought about this a bit more and it is wrong. 4^^4 is about 10^10^154. A number with 50% more digits than a googolplex would be about
10^(1.5*10^100), so far less than 10^10^101 (which would have 10 times as many digits as a googolplex). The number 4^^4 is about a googolplex to the power of 10^50, so (googolplex)^\sqrt(googol)
By the way, 10^^10 is called Decker.
Why are people so impressed by the number of fingers on their hands? 9^^9 is so much more elegant.
If you want to be hateful you just take one complex irrational number and raise it to the power of another complex irrational. Or to the superpower if you want to get really nasty.
Posts: 1307
Threads: 3
Joined: November 16, 2018
Reputation:
18
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 10, 2020 at 2:34 am
(June 9, 2020 at 1:44 pm)polymath257 Wrote: (June 9, 2020 at 12:42 am)Paleophyte Wrote: In practice, people tend to be exceptionally bad at properly examining the probability space. Probabilities lower than 1 in a googolplex happen every instant of our lives but we fail to recognize them because of the stochastic nature of the universe that we inhabit. In the instance of the egg the rational course of action is not to start worshipping Gawd AllMighty Mender Of The Yolk but rather to look for the Gallifreyan pankster who has been unscrambling your omelettes.
On a side note, googol and googolplex have always failed to impress me. They're stunt numbers based on the number of fingers on your hands. If really big numbers is all you want then 4^^4 is a bit better than 50% more digits than a googolplex and 9^^9 should be more than sufficient to tie up any computer from now until the end of time.
I think you might find it more difficult than you imagine to get odds of 1 in a googolplex.
So, for example, the radius of the observable universe is about 13 billion light years
46.5 billion light years last I checked.
Quote:which is around 10^26 meters, or 10^38 femto-meters.
So, the number of cubic femtometers in the observable universe is around 10^114.
The number of fundamental particles in the universe is around 10^80, so the odds that the specific arrangement of particles in the space of the universe (up to femtometer accuracy) is about (10^114)^(10^80), which is less than 10^(10^83). This is assuming the position of each particle is independent of every other particle. This is *far* less than a gogolplex.
Now, the odds for every fundamental particle in the universe *randomly* and independently happening to be in the specific cubic femtometer they are, independently for each femtosecond n a second, would be less than (10^10^83)^(10^12), which is about 10^10^95. This is still far smaller than a googolplex.
In fact, one in a googolplex would be worse odds than the odds of every particle in the universe randomly and independently being in the precise cubic femtometer, independently for each femtosecond in 100,000 years.
So, no, we do NOT see events with a lower probability happening every instant of our lives.
Not when you math it like that, no. However:
- Femtometer resolution is ridiculously coarse right out of the gate. You won't even be able to predict if two protons are on course to fuse into a deuteron or not at that resolution.
- You've neglected to account for all the various different properties that each of those fundamental particles can have. Those are going to be important.
- The whole mess is iterative, which means that each ridiculously improbable configuration follows from an equally preposterous configuration. That means that if you don't have extremely good resolution the errors compound very quickly. Given that the large-scale structure of the universe originated as quantum fluctuations during the universe' inflationary era you're probably going to need Planck-scale resolution in all four dimensions.
If you want to know the probability of these exact sodium ions being pumped in and out of my neurons, to send exactly these electrons and photons bouncing around the pinball game we call the internet, to eventually light up exactly these atoms in your display, trigger precisely these opsins, and fire exactly these sodium-ion gates in your neurons, yadda, yadda, yadda, blah, blah, blah... requires calculating all the intermediate and preceding states and their improbabilities. If just one proton had been a bit to one side rather than the other 10 billion years ago then stars in our distant history would have blown themselves apart very slightly differently, our constituent atoms would never have been formed, and somebody very different would be having a very different conversation.
Quote:PS: We *do* see events with probabilities lower than 1 in a googol every instant. But a googolplex is much, much, much larger than a googol.
Yes, you can beat a 1 in a googol if you have $3.33 in pennies and that's doing it the lazy way. Or a couple well-shuffled decks of cards.
Posts: 2417
Threads: 5
Joined: January 3, 2018
Reputation:
22
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 10, 2020 at 7:23 am
(This post was last modified: June 10, 2020 at 7:56 am by polymath257.)
(June 10, 2020 at 2:34 am)Paleophyte Wrote: (June 9, 2020 at 1:44 pm)polymath257 Wrote: I think you might find it more difficult than you imagine to get odds of 1 in a googolplex.
So, for example, the radius of the observable universe is about 13 billion light years
46.5 billion light years last I checked.
13 billion is the farther distance we can see. 46 billion is the current distance of those things that took 13 billion years for the light to get to us.
The difference, one this scale, isn't particularly relevant: maybe another factor of 100.
Quote:Quote:which is around 10^26 meters, or 10^38 femto-meters.
So, the number of cubic femtometers in the observable universe is around 10^114.
The number of fundamental particles in the universe is around 10^80, so the odds that the specific arrangement of particles in the space of the universe (up to femtometer accuracy) is about (10^114)^(10^80), which is less than 10^(10^83). This is assuming the position of each particle is independent of every other particle. This is *far* less than a gogolplex.
Now, the odds for every fundamental particle in the universe *randomly* and independently happening to be in the specific cubic femtometer they are, independently for each femtosecond n a second, would be less than (10^10^83)^(10^12), which is about 10^10^95. This is still far smaller than a googolplex.
In fact, one in a googolplex would be worse odds than the odds of every particle in the universe randomly and independently being in the precise cubic femtometer, independently for each femtosecond in 100,000 years.
So, no, we do NOT see events with a lower probability happening every instant of our lives.
Not when you math it like that, no. However:
- Femtometer resolution is ridiculously coarse right out of the gate. You won't even be able to predict if two protons are on course to fuse into a deuteron or not at that resolution.
- You've neglected to account for all the various different properties that each of those fundamental particles can have. Those are going to be important.
- The whole mess is iterative, which means that each ridiculously improbable configuration follows from an equally preposterous configuration. That means that if you don't have extremely good resolution the errors compound very quickly. Given that the large-scale structure of the universe originated as quantum fluctuations during the universe' inflationary era you're probably going to need Planck-scale resolution in all four dimensions.
If you want to know the probability of these exact sodium ions being pumped in and out of my neurons, to send exactly these electrons and photons bouncing around the pinball game we call the internet, to eventually light up exactly these atoms in your display, trigger precisely these opsins, and fire exactly these sodium-ion gates in your neurons, yadda, yadda, yadda, blah, blah, blah... requires calculating all the intermediate and preceding states and their improbabilities. If just one proton had been a bit to one side rather than the other 10 billion years ago then stars in our distant history would have blown themselves apart very slightly differently, our constituent atoms would never have been formed, and somebody very different would be having a very different conversation.
And if you do Planck length accuracy that 10^38 turns into 10^70, so the volume turns into 10^210. The approximation I made at that point put the volume less than 10^1000. The rest was taken into account in my calculation. Yes, that 1 proton difference was taken into account by my raising things to the power of the number of particles in the universe. Each different arrangement would be different and I picked out the one that it actually is.
The other properties, again, add a few to the exponent but won't get it up to a googol.
Quote:Quote:PS: We *do* see events with probabilities lower than 1 in a googol every instant. But a googolplex is much, much, much larger than a googol.
Yes, you can beat a 1 in a googol if you have $3.33 in pennies and that's doing it the lazy way. Or a couple well-shuffled decks of cards.
Again, like I said, we see odds of 1 in a googol all the time. We do NOT see odds of 1 in a googolplex all the time. Remember that a googolplex is 10^googol and a googol is a big number compared to the size of the universe or the number of particles in it.
(June 10, 2020 at 12:53 am)Paleophyte Wrote: (June 9, 2020 at 6:59 pm)polymath257 Wrote: I thought about this a bit more and it is wrong. 4^^4 is about 10^10^154. A number with 50% more digits than a googolplex would be about
10^(1.5*10^100), so far less than 10^10^101 (which would have 10 times as many digits as a googolplex). The number 4^^4 is about a googolplex to the power of 10^50, so (googolplex)^\sqrt(googol)
By the way, 10^^10 is called Decker.
Why are people so impressed by the number of fingers on their hands? 9^^9 is so much more elegant.
If you want to be hateful you just take one complex irrational number and raise it to the power of another complex irrational. Or to the superpower if you want to get really nasty.
Or, instead of tetration, you can do higher order operations. Say, 4^^^^4, or 9^^^^^^^^^9. Those are both MUCH larger.
But the googology site still considers those to be *small*.
I'm not sure why you think a complex irrational to the power of a complex irrational has to be large at all. For example, e^(i\pi) =-1, famously.
Posts: 1307
Threads: 3
Joined: November 16, 2018
Reputation:
18
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 10, 2020 at 8:59 pm
(June 10, 2020 at 7:23 am)polymath257 Wrote: And if you do Planck length accuracy that 10^38 turns into 10^70, so the volume turns into 10^210. The approximation I made at that point put the volume less than 10^1000. The rest was taken into account in my calculation. Yes, that 1 proton difference was taken into account by my raising things to the power of the number of particles in the universe. Each different arrangement would be different and I picked out the one that it actually is.
The other properties, again, add a few to the exponent but won't get it up to a googol.
I'm not convinced that you're mathing it right.
A standard deck of playing cards has 52 cards once you remove the jokers, so there are 52! or ~ 8*10 67 possible combinations, each of which is equally unlikely.
One mole of water is 18 grams, or just a bit more than a tablespoon at STP. A random arrangement of the molecules produces 6.022*10 23! possibilities, which is a bit better than 10^10 25. That isn't a googolplex but it's headed the right direction and we've gotten that far by just by arranging the molecules in a simple random array. It doesn't take into account any of the properties of the water molecules or their interactions and certainly doesn't account for iteration over time. Factoring in space at nanometer scale and time on a nanosecond scale seems to yield ~10^10 55 possible arrangements per mole per second. Given that we still haven't factored in a lot of the properties of the molecules or any of their interactions it seems likely that we can beat googolplex odds with a pint of beer. If I haven't mathed it wrong, which is very likely.
The observable universe has roughly 10 80 fundamental particles and the factorial of that is just shy of 10^10 82. Still not a googolplex but much closer and that is just lining the particles up randomly with no accounting for spatial or temporal resolution, much less any of the more interesting attributes and interactions of some of those particles. Given that many permutations as a starting point and adding in spatial resolution at even the light year scale would appear to topple googolpex odds every moment of our existence.
Quote:I'm not sure why you think a complex irrational to the power of a complex irrational has to be large at all. For example, e^(i\pi) =-1, famously.
I wasn't trying to make it big, I was trying to make it horrible. In some very special rare cases it resolves to a sane number but typically you end up with horrid endless series of fractions. Like this little horror:
![[Image: mc3bx59.gif]](https://i.imgur.com/mc3bx59.gif)
Posts: 2417
Threads: 5
Joined: January 3, 2018
Reputation:
22
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 11, 2020 at 8:21 am
(This post was last modified: June 11, 2020 at 8:31 am by polymath257.)
(June 10, 2020 at 8:59 pm)Paleophyte Wrote: (June 10, 2020 at 7:23 am)polymath257 Wrote: And if you do Planck length accuracy that 10^38 turns into 10^70, so the volume turns into 10^210. The approximation I made at that point put the volume less than 10^1000. The rest was taken into account in my calculation. Yes, that 1 proton difference was taken into account by my raising things to the power of the number of particles in the universe. Each different arrangement would be different and I picked out the one that it actually is.
The other properties, again, add a few to the exponent but won't get it up to a googol.
I'm not convinced that you're mathing it right.
A standard deck of playing cards has 52 cards once you remove the jokers, so there are 52! or ~ 8*1067 possible combinations, each of which is equally unlikely.
And in my estimate, I was *underestimating* the factorial by using n!<n^n. In the case of a deck of cards, 52! <52^52 <100^52 =10^104. A rather big over-estimate of the factorial, I think.
Quote:One mole of water is 18 grams, or just a bit more than a tablespoon at STP. A random arrangement of the molecules produces 6.022*1023! possibilities, which is a bit better than 10^1025. That isn't a googolplex but it's headed the right direction and we've gotten that far by just by arranging the molecules in a simple random array. It doesn't take into account any of the properties of the water molecules or their interactions and certainly doesn't account for iteration over time. Factoring in space at nanometer scale and time on a nanosecond scale seems to yield ~10^1055 possible arrangements per mole per second. Given that we still haven't factored in a lot of the properties of the molecules or any of their interactions it seems likely that we can beat googolplex odds with a pint of beer. If I haven't mathed it wrong, which is very likely.
NO, That is NOWHERE close to a googolplex. It barely scratches the surface, in fact. Now try (10^80)!, which is less than (10^80)^(10^80), which is less than 10^10^83.
Let's do nanometer scale for a second at nanosecond scale with a pint of beer (water). Now, a pint is approximately half a liter, which is 500cc. There is 1 gram of water per cc, and the molecular weight of water is 18, so there are 500/18 <30 moles of water in a pint. This gives 30*6*10^23<2*10^25 <10^26 molecules of water.
We'll call 500cc~500*10^21 =5*10^23 cubic namometers. And a second is 10^9 ns. That gives5* 10^32<10^33 nm.ns in a pint of beer for a second.
Notice that at each stage I over-estimated the numbers involved, sometimes quite drastically.
Next, the number of possible arrangements of 10^26 molecules is, as you say, (10^26)!<(10^26)^(10^26) <(10^100)^(10^26)=10^(100*10^26)=10^10^28.
Again, the estimate of 10^26 <10^100 is a HUGE over-estimate as is the estimate (10^26)! <(10^26)^(10^26). In general n! <n^n.
Next, let's do (10^10^28)^(10^33) for a random arrangement for each nanometer.nanosecond. This gives
(10^10^28)^(10^33) =10^(10^28 * 10^33)=10^10^61 possibilities.
This is still a LONG way away from a googolplex.
Quote:The observable universe has roughly 1080 fundamental particles and the factorial of that is just shy of 10^1082. Still not a googolplex but much closer and that is just lining the particles up randomly with no accounting for spatial or temporal resolution, much less any of the more interesting attributes and interactions of some of those particles. Given that many permutations as a starting point and adding in spatial resolution at even the light year scale would appear to topple googolpex odds every moment of our existence.
The problem is that expressions like (10^n)! <(10^n)^(10*n)=10^(n*10^n) don't tend to increase the second exponent by much. For n<100, you get AT MOST 10^10^(n+2).
You can add another 7 to that second exponent by considering that pint for a full year. And then another 22 or so if you consider the whole earth. That gets you up to 10^10^90, but I think that already violates your original claim that we see such odds all the time.
Now, if you do femtometers and femtoseconds for the whole Earth for a full year, you can, indeed get above a googolplex, managing odds of 1 in 10^10^102.
Good luck getting to 10^10^200. *grin*
Quote:Quote:I'm not sure why you think a complex irrational to the power of a complex irrational has to be large at all. For example, e^(i\pi) =-1, famously.
I wasn't trying to make it big, I was trying to make it horrible. In some very special rare cases it resolves to a sane number but typically you end up with horrid endless series of fractions. Like this little horror:
![[Image: mc3bx59.gif]](https://i.imgur.com/mc3bx59.gif)
Hmmm....the main difficulty with complex exponentials is that they are multiple valued. The complex number in the base has infinitely many logarithms, so the definition of the expression gives infinitely many different values, depending on which value of the logarithm you use. It isn't going to simplify algebraically in any nice way (but neither does sqrt(2)^sqrt(2) ).
Nonetheless, we can say that all values are transcendental numbers (not the root of any polynomial with integer coefficients).
Posts: 1307
Threads: 3
Joined: November 16, 2018
Reputation:
18
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 12, 2020 at 3:16 am
(June 11, 2020 at 8:21 am)polymath257 Wrote: (June 10, 2020 at 8:59 pm)Paleophyte Wrote: I'm not convinced that you're mathing it right.
A standard deck of playing cards has 52 cards once you remove the jokers, so there are 52! or ~ 8*1067 possible combinations, each of which is equally unlikely.
And in my estimate, I was *underestimating* the factorial by using n!<n^n. In the case of a deck of cards, 52! <52^52 <100^52 =10^104. A rather big over-estimate of the factorial, I think.
Quote:One mole of water is 18 grams, or just a bit more than a tablespoon at STP. A random arrangement of the molecules produces 6.022*1023! possibilities, which is a bit better than 10^1025. That isn't a googolplex but it's headed the right direction and we've gotten that far by just by arranging the molecules in a simple random array. It doesn't take into account any of the properties of the water molecules or their interactions and certainly doesn't account for iteration over time. Factoring in space at nanometer scale and time on a nanosecond scale seems to yield ~10^1055 possible arrangements per mole per second. Given that we still haven't factored in a lot of the properties of the molecules or any of their interactions it seems likely that we can beat googolplex odds with a pint of beer. If I haven't mathed it wrong, which is very likely.
NO, That is NOWHERE close to a googolplex. It barely scratches the surface, in fact. Now try (10^80)!, which is less than (10^80)^(10^80), which is less than 10^10^83.
Let's do nanometer scale for a second at nanosecond scale with a pint of beer (water). Now, a pint is approximately half a liter, which is 500cc. There is 1 gram of water per cc, and the molecular weight of water is 18, so there are 500/18 <30 moles of water in a pint. This gives 30*6*10^23<2*10^25 <10^26 molecules of water.
We'll call 500cc~500*10^21 =5*10^23 cubic namometers. And a second is 10^9 ns. That gives5* 10^32<10^33 nm.ns in a pint of beer for a second.
Notice that at each stage I over-estimated the numbers involved, sometimes quite drastically.
Next, the number of possible arrangements of 10^26 molecules is, as you say, (10^26)!<(10^26)^(10^26) <(10^100)^(10^26)=10^(100*10^26)=10^10^28.
Again, the estimate of 10^26 <10^100 is a HUGE over-estimate as is the estimate (10^26)! <(10^26)^(10^26). In general n! <n^n.
Next, let's do (10^10^28)^(10^33) for a random arrangement for each nanometer.nanosecond. This gives
(10^10^28)^(10^33) =10^(10^28 * 10^33)=10^10^61 possibilities.
This is still a LONG way away from a googolplex.
Quote:The observable universe has roughly 1080 fundamental particles and the factorial of that is just shy of 10^1082. Still not a googolplex but much closer and that is just lining the particles up randomly with no accounting for spatial or temporal resolution, much less any of the more interesting attributes and interactions of some of those particles. Given that many permutations as a starting point and adding in spatial resolution at even the light year scale would appear to topple googolpex odds every moment of our existence.
The problem is that expressions like (10^n)! <(10^n)^(10*n)=10^(n*10^n) don't tend to increase the second exponent by much. For n<100, you get AT MOST 10^10^(n+2).
You can add another 7 to that second exponent by considering that pint for a full year. And then another 22 or so if you consider the whole earth. That gets you up to 10^10^90, but I think that already violates your original claim that we see such odds all the time.
Now, if you do femtometers and femtoseconds for the whole Earth for a full year, you can, indeed get above a googolplex, managing odds of 1 in 10^10^102.
OK, check my thinking on this:
n! doesn't represent the probabilities correctly. That's just the number of permutations you'd get if you shuffled the system's components and put them into a linear array like a deck of cards. We need to account for spatial distribution because there's a big difference between two molecules a light year apart and the same two molecules a micrometer apart.
To account for space we need something more like a 'seat n people in m chairs' problem but seating particles in potential locations. That yields m!/(m-n)! combinations, which can be nasty to calculate. Happily, so long as the number of potential locations is much, much larger than the number of particles, the difference between m and m-n is trivial and we can approximate it as m^n, which is a much simpler calculation. It's simpler than that since, as you pointed out, the value of m doesn't influence the uppermost exponent much. The spatial resolution doesn't matter much so long as it's fine enough to satisfy m >> n. In that case the calculations get really simple because a system with 10^x components has roughly 10^10^x+2 possible arrangements. So a pint of water with ~10^25 molecules wouldn't have more than 10^10^27 possible arrangements, which is impressively large but a long way short of a googolplex.
But none of that accounts for time. We need to set this all in motion so what we need is less of a 'seat n people in m chairs' problem and more of a 'musical chairs' problem but with a lot more chairs than people. For this we simply view each iteration as a single m^n snapshot and multiply the results for i iterations. That will be valid so long as each m^n snapshot is unique, which it will be since the laws of thermodymanics preclude take-backsies. That produces m^n^i possible combinations, and that will get very large very quickly.
Using nanosecond resolution, a pint of water with 10^25 molecules passes the googolplex mark in 4 nanoseconds, beats 10^10^200 in 8 nanoseconds, and passes 10^10^25,000,000,000 in the first second. I know, those are absurdly large numbers, but that's what iterating in 4 dimensions gets you.
Quote:Quote:I wasn't trying to make it big, I was trying to make it horrible. In some very special rare cases it resolves to a sane number but typically you end up with horrid endless series of fractions. Like this little horror:
![[Image: mc3bx59.gif]](https://i.imgur.com/mc3bx59.gif)
Hmmm....the main difficulty with complex exponentials is that they are multiple valued. The complex number in the base has infinitely many logarithms, so the definition of the expression gives infinitely many different values, depending on which value of the logarithm you use. It isn't going to simplify algebraically in any nice way (but neither does sqrt(2)^sqrt(2) ).
Nonetheless, we can say that all values are transcendental numbers (not the root of any polynomial with integer coefficients).
They spawn an infinity of little horrors. 
Posts: 2417
Threads: 5
Joined: January 3, 2018
Reputation:
22
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 12, 2020 at 7:30 am
(This post was last modified: June 12, 2020 at 7:39 am by polymath257.)
(June 12, 2020 at 3:16 am)Paleophyte Wrote: (June 11, 2020 at 8:21 am)polymath257 Wrote: And in my estimate, I was *underestimating* the factorial by using n!<n^n. In the case of a deck of cards, 52! <52^52 <100^52 =10^104. A rather big over-estimate of the factorial, I think.
NO, That is NOWHERE close to a googolplex. It barely scratches the surface, in fact. Now try (10^80)!, which is less than (10^80)^(10^80), which is less than 10^10^83.
Let's do nanometer scale for a second at nanosecond scale with a pint of beer (water). Now, a pint is approximately half a liter, which is 500cc. There is 1 gram of water per cc, and the molecular weight of water is 18, so there are 500/18 <30 moles of water in a pint. This gives 30*6*10^23<2*10^25 <10^26 molecules of water.
We'll call 500cc~500*10^21 =5*10^23 cubic namometers. And a second is 10^9 ns. That gives5* 10^32<10^33 nm.ns in a pint of beer for a second.
Notice that at each stage I over-estimated the numbers involved, sometimes quite drastically.
Next, the number of possible arrangements of 10^26 molecules is, as you say, (10^26)!<(10^26)^(10^26) <(10^100)^(10^26)=10^(100*10^26)=10^10^28.
Again, the estimate of 10^26 <10^100 is a HUGE over-estimate as is the estimate (10^26)! <(10^26)^(10^26). In general n! <n^n.
Next, let's do (10^10^28)^(10^33) for a random arrangement for each nanometer.nanosecond. This gives
(10^10^28)^(10^33) =10^(10^28 * 10^33)=10^10^61 possibilities.
This is still a LONG way away from a googolplex.
The problem is that expressions like (10^n)! <(10^n)^(10*n)=10^(n*10^n) don't tend to increase the second exponent by much. For n<100, you get AT MOST 10^10^(n+2).
You can add another 7 to that second exponent by considering that pint for a full year. And then another 22 or so if you consider the whole earth. That gets you up to 10^10^90, but I think that already violates your original claim that we see such odds all the time.
Now, if you do femtometers and femtoseconds for the whole Earth for a full year, you can, indeed get above a googolplex, managing odds of 1 in 10^10^102.
OK, check my thinking on this:
n! doesn't represent the probabilities correctly. That's just the number of permutations you'd get if you shuffled the system's components and put them into a linear array like a deck of cards. We need to account for spatial distribution because there's a big difference between two molecules a light year apart and the same two molecules a micrometer apart.
To account for space we need something more like a 'seat n people in m chairs' problem but seating particles in potential locations. That yields m!/(m-n)! combinations, which can be nasty to calculate. Happily, so long as the number of potential locations is much, much larger than the number of particles, the difference between m and m-n is trivial and we can approximate it as m^n, which is a much simpler calculation. It's simpler than that since, as you pointed out, the value of m doesn't influence the uppermost exponent much. The spatial resolution doesn't matter much so long as it's fine enough to satisfy m >> n. In that case the calculations get really simple because a system with 10^x components has roughly 10^10^x+2 possible arrangements. So a pint of water with ~10^25 molecules wouldn't have more than 10^10^27 possible arrangements, which is impressively large but a long way short of a googolplex.
But none of that accounts for time. We need to set this all in motion so what we need is less of a 'seat n people in m chairs' problem and more of a 'musical chairs' problem but with a lot more chairs than people. For this we simply view each iteration as a single m^n snapshot and multiply the results for i iterations. That will be valid so long as each m^n snapshot is unique, which it will be since the laws of thermodymanics preclude take-backsies. That produces m^n^i possible combinations, and that will get very large very quickly.
No, it produces (m^n)^i =m^(ni). For this, you *multiply* exponents. This is very different than m^n^i=m^(n^i). You are doing the parentheses the wrong way and it makes a difference. (x^y)^z=x^(yz) is very different than x^(y^z).
For example, (10^3)^2 =(1000)^2=1000000=10^6, but 10^(3^2)=10^9. Or, another, (10^3)^10=10^30, but 10^3^10 =10^59049, which is considerably bigger.
Quote:Using nanosecond resolution, a pint of water with 10^25 molecules passes the googolplex mark in 4 nanoseconds, beats 10^10^200 in 8 nanoseconds, and passes 10^10^25,000,000,000 in the first second. I know, those are absurdly large numbers, but that's what iterating in 4 dimensions gets you.
No, it passes through the *googol* mark in 4 nanoseconds (10^25)^4=10^100. That is a googol. It gets to 10^200 in 8 seconds. In a second, it gets to (10^25)^(10^9) =10^(25*10^9)<10^(100*10^9)=10^10^11
In a year, it would get to (10^25)^(3*10^16)=10^(75*10^16)<10^(100*10^16)=10^10^18
In 10 billion years, it would get to (10^25)^(3*10^26)<10^10^28
Quote:Quote:Hmmm....the main difficulty with complex exponentials is that they are multiple valued. The complex number in the base has infinitely many logarithms, so the definition of the expression gives infinitely many different values, depending on which value of the logarithm you use. It isn't going to simplify algebraically in any nice way (but neither does sqrt(2)^sqrt(2) ).
Nonetheless, we can say that all values are transcendental numbers (not the root of any polynomial with integer coefficients).
They spawn an infinity of little horrors. 
Some people enjoy such horrors. I'm a professional mathematician. This is my wheelhouse.
Posts: 1307
Threads: 3
Joined: November 16, 2018
Reputation:
18
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 12, 2020 at 7:05 pm
(June 12, 2020 at 7:30 am)polymath257 Wrote: (June 12, 2020 at 3:16 am)Paleophyte Wrote: OK, check my thinking on this:
n! doesn't represent the probabilities correctly. That's just the number of permutations you'd get if you shuffled the system's components and put them into a linear array like a deck of cards. We need to account for spatial distribution because there's a big difference between two molecules a light year apart and the same two molecules a micrometer apart.
To account for space we need something more like a 'seat n people in m chairs' problem but seating particles in potential locations. That yields m!/(m-n)! combinations, which can be nasty to calculate. Happily, so long as the number of potential locations is much, much larger than the number of particles, the difference between m and m-n is trivial and we can approximate it as m^n, which is a much simpler calculation. It's simpler than that since, as you pointed out, the value of m doesn't influence the uppermost exponent much. The spatial resolution doesn't matter much so long as it's fine enough to satisfy m >> n. In that case the calculations get really simple because a system with 10^x components has roughly 10^10^x+2 possible arrangements. So a pint of water with ~10^25 molecules wouldn't have more than 10^10^27 possible arrangements, which is impressively large but a long way short of a googolplex.
But none of that accounts for time. We need to set this all in motion so what we need is less of a 'seat n people in m chairs' problem and more of a 'musical chairs' problem but with a lot more chairs than people. For this we simply view each iteration as a single m^n snapshot and multiply the results for i iterations. That will be valid so long as each m^n snapshot is unique, which it will be since the laws of thermodymanics preclude take-backsies. That produces m^n^i possible combinations, and that will get very large very quickly.
No, it produces (m^n)^i =m^(ni). For this, you *multiply* exponents. This is very different than m^n^i=m^(n^i). You are doing the parentheses the wrong way and it makes a difference. (x^y)^z=x^(yz) is very different than x^(y^z).
For example, (10^3)^2 =(1000)^2=1000000=10^6, but 10^(3^2)=10^9. Or, another, (10^3)^10=10^30, but 10^3^10 =10^59049, which is considerably bigger.
Thanx, you're right, I got my brackets wrong. m ni
Quote:Quote:Using nanosecond resolution, a pint of water with 10^25 molecules passes the googolplex mark in 4 nanoseconds, beats 10^10^200 in 8 nanoseconds, and passes 10^10^25,000,000,000 in the first second. I know, those are absurdly large numbers, but that's what iterating in 4 dimensions gets you.
No, it passes through the *googol* mark in 4 nanoseconds (10^25)^4=10^100. That is a googol. It gets to 10^200 in 8 seconds. In a second, it gets to (10^25)^(10^9) =10^(25*10^9)<10^(100*10^9)=10^10^11
In a year, it would get to (10^25)^(3*10^16)=10^(75*10^16)<10^(100*10^16)=10^10^18
In 10 billion years, it would get to (10^25)^(3*10^26)<10^10^28
Errr, no. A single m n snapshot gives you 10^10^27, which has left googol so far behind in the dust that it's laughable. That said, this won't get my beer over the googolplex line at m ni for any reasonable value of i. At nanosecond resolution you're looking at 10^46 times the age of the universe. Even at Planck time resolution you're looking at a triilion times the age of the universe or so. So I can't get my beer over the googolplex mark, at least not using this simplistic analysis of the odds. I'm still hopeful that factoring in all the other complexities would get it over the line but I don't have the mathing for that.
The universe however gets us ~10^10^82 permutations per snapshot. That means that we need 10^18 iterations to get us over the googolplex mark or, attosecond resolution to achieve googolplex per second. Attosecond resolution is pretty reasonable given that it's the timescale at which helium is fused into carbon and you could hide the entire inflationary epoch inside of it. And since the fundamental structure of our universe was established in those first seconds, we experience events of 1 in a googolplex every moment of our lives.
Quote:Quote:They spawn an infinity of little horrors. 
Some people enjoy such horrors. I'm a professional mathematician. This is my wheelhouse.
Sorry, text fails to convey the admiration that I feel for said little horrors.
Posts: 70
Threads: 0
Joined: June 13, 2020
Reputation:
0
RE: Is it ever physically possible for a broken egg to reassemble into an unbroken one?
June 13, 2020 at 5:52 am
I don't understand the question. 'resemble into'?
If you're asking if it's ever possible for an unbroken egg to be broken then ......... no.
|



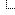


![[Image: mc3bx59.gif]](https://i.imgur.com/mc3bx59.gif)